Learning Outcomes
- Define atomic and mass numbers.
- Determine the number of protons, neutrons, and electrons in an atom.
- Identify the charge and relative mass of subatomic particles.
- Label the location of subatomic particles in the atom.
- Determine the mass of an atom based on its subatomic particles.
- Write A/Z and symbol-mass format for an atom.
The atomic number is the number of protons in the nucleus of an atom. The number of protons define the identity of an element (i.e., an element with 6 protons is a carbon atom, no matter how many neutrons may be present). The atomic number is the number that determines the identity of an atom, that is, the element to which that atom belongs. On the other hand, a mass number is a number that is used to compare. Atomic Number The atomic number or proton number is defined as the total number of protons in the nucleus and is given the symbol Z. The number of electrons in an electrically-neutral atom is the same as the atomic number. The total electrical charge of the nucleus is therefore +Ze. Atomic number is defined as the number of protons in the nucleus of an atom. Elements are identified based on the number protons in their nucleus. Any atom with 2 protons is called a Helium atom. Has an atomic number double that of sodium’s. Has the lowest atomic number of any group 15 element. Is in group 1; has a higher atomic number than Cl but a lower atomic number than Br. Has between 35 and 55 protons and is in group 17. Has the lowest atomic mass on the periodic table.
Atoms are the fundamental building blocks of all matter and are composed of protons, neutrons, and electrons. Because atoms are electrically neutral, the number of positively charged protons must be equal to the number of negatively charged electrons. Since neutrons do not affect the charge, the number of neutrons is not dependent on the number of protons and will vary even among atoms of the same element.
Atomic Number
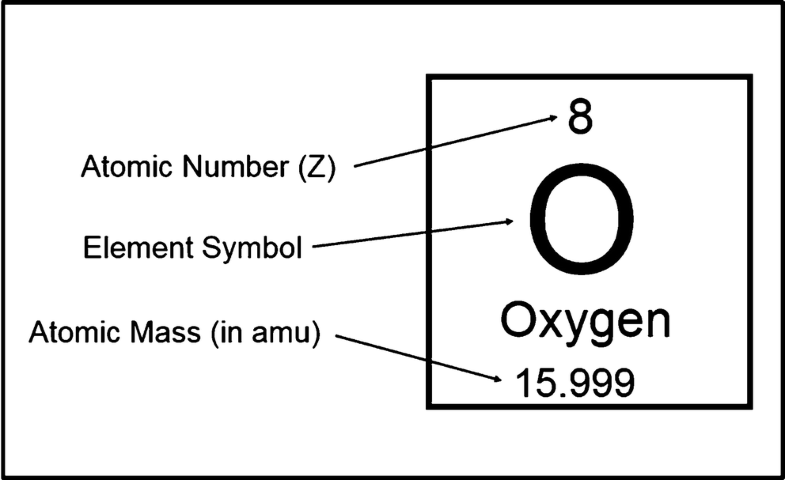
The atomic number (represented by the letter Z)of an element is the number of protons in the nucleus of each atom of that element. An atom can be classified as a particular element based solely on its atomic number. For example, any atom with an atomic number of 8 (its nucleus contains 8 protons) is an oxygen atom, and any atom with a different number of protons would be a different element. The periodic table (see figure below) displays all of the known elements and is arranged in order of increasing atomic number. In this table, an element's atomic number is indicated above the elemental symbol. Hydrogen, at the upper left of the table, has an atomic number of 1. Every hydrogen atom has one proton in its nucleus. Next on the table is helium, whose atoms have two protons in the nucleus. Lithium atoms have three protons, beryllium atoms have four, and so on.
Since atoms are neutral, the number of electrons in an atom is equal to the number of protons. Hydrogen atoms all have one electron occupying the space outside of the nucleus. Helium, with two protons, will have two electrons. In the chemical classroom, the proton count will always be equivalent to an atom's atomic number. This value will not change unless the nucleus decays or is bombarded (nuclear physics).
Mass Number
Experimental data showed that the vast majority of the mass of an atom is concentrated in its nucleus, which is composed of protons and neutrons. The mass number (represented by the letter A)is defined as the total number of protons and neutrons in an atom. Consider the table below, which shows data from the first six elements of the periodic table.
| Name | Symbol | Atomic Number (Z) | Protons | Neutrons | Electrons | Mass Number (A) (rounded to two decimals) |
|---|---|---|---|---|---|---|
| hydrogen | (ce{H}) | 1 | 1 | 0 | 1 | 1.01 |
| helium | (ce{He}) | 2 | 2 | 2 | 2 | 4.00 |
| lithium | (ce{Li}) | 3 | 3 | 4 | 3 | 6.94 |
| beryllium | (ce{Be}) | 4 | 4 | 5 | 4 | 9.01 |
| boron | (ce{B}) | 5 | 5 | 6 | 5 | 10.18 |
| carbon | (ce{C}) | 6 | 6 | 6 | 6 | 12.01 |
Consider the element helium. Its atomic number is 2, so it has two protons in its nucleus. Its nucleus also contains two neutrons. Since (2 + 2 = 4), we know that the mass number of the helium atom is 4. Finally, the helium atom also contains two electrons, since the number of electrons must equal the number of protons. This example may lead you to believe that atoms have the same number of protons and neutrons, but a further examination of the table above will show that this is not the case. Lithium, for example, has three protons and four neutrons, giving it a mass number of 7.
Knowing the mass number and the atomic number of an atom allows you to determine the number of neutrons present in that atom by subtraction.
[text{Number of neutrons} = text{ rounded mass number} - text{atomic number}]
Atoms of the element chromium (left( ce{Cr} right)) have an atomic number of 24 and a mass number of 52. How many neutrons are in the nucleus of a chromium atom? To determine this, you would subtract as shown:
[52 - 24 = 28 : text{neutrons in a chromium atom}]
The composition of any atom can be illustrated with a shorthand notation called A/Z format. Both the atomic number and mass are written to the left of the chemical symbol. The 'A' value is written as a superscript while the 'Z' value is written as a subscript. For an example of this notation, look to the chromium atom shown below:
[ce{^{52}_{24}Cr}]
Another way to refer to a specific atom is to write the mass number of the atom after the name, separated by a hyphen. Symbol-mass format for the above atom would be written as Cr-52. In this notation, the atomic number is not included. You will need to refer to a periodic table for proton values.
Example (PageIndex{1})
Calculate each of the three subatomic particles and give specific group or period names for each atom.
- mercury
- platinum
- bromine
Solutions
- Hg (transition metal)- has 80 electrons, 80 protons, and 121 neutrons
- Pt (transition metal)- has 78 electrons, 78 protons, and 117 neutrons
- Br (halogen)- has 35 electrons, 35 protons, and 45 neutrons
Example (PageIndex{2})
Write both A/Z and symbol-mass formats for the atoms in Example (PageIndex{1}).
Solutions
- (ce{^{201}_{80}Hg}) and Hg-201
- (ce{^{195}_{78}Pt}) and Pt-195
- (ce{^{80}_{35}Br}) and Br-80
Example (PageIndex{3})
Identify the elements based on the statements below.
- Which element has 25 protons?
- Which element has 0 neutrons?
- Which element has 83 electrons?
Solutions
Atomic Number Chart
a. manganese
b. hydrogen
c. bismuth
Need More Practice?
- Turn to section 3.E of this OER and answer questions #1-#2, #4, and #8.
Contributors
CK-12 Foundation by Sharon Bewick, Richard Parsons, Therese Forsythe, Shonna Robinson, and Jean Dupon.
Allison Soult, Ph.D. (Department of Chemistry, University of Kentucky)
We remember from our school chemistry course that every element has its own specific atomic number. It is the same as the number of protons that the atom of each element has, so sometimes atomic number is called proton number. It is always the whole number and it ranges from 1 to 118, according to the number of the element in the Periodic Table. This number can be really important and something essential to know, in relation to a certain chemical element which is the issue of our interest at the moment.
Why is this so? Why is the atomic number so important? First of all, it is the number that makes elements different from one another as it shows the number of protons in their nuclei. Also, knowing the atomic number of an element can give us an idea about the position of the element in the Periodic Table. Atomic number of an element never changes: for example, the atomic number of oxygen is always 8, and the atomic number of Chlorine is always 18. The atomic number is marked with the symbol Z, taken from a German word zahl (or atomzahl, which is 'atomic number' in German).
This website is created for those who need to know the atomic number of a central chemical element. By using our website, you can do it in just one click and receive short and correct information on this matter. There is also some extra summary on every each chemical element which can be found at our website, including the atomic weight of each element, as well as physical and chemical properties of every element and its importance. Use this website at any time when you need to get fast and precise information about atomic or proton number of chemical elements.
List of chemical elements in periodic table with atomic number, chemical symbol and atomic weight. You can sort the elements by clicking on the table headers. Please click on the element name for complete list of element properties.
Definition Of Atomic Number
| Atomic Number | Chemical Symbol | Element Name | Atomic Weight (u) |
|---|---|---|---|
| 1 | H | Hydrogen | 1.008 |
| 2 | He | Helium | 4.003 |
| 3 | Li | Lithium | 6.94 |
| 4 | Be | Beryllium | 9.012 |
| 5 | B | Boron | 10.81 |
| 6 | C | Carbon | 12.011 |
| 7 | N | Nitrogen | 14.007 |
| 8 | O | Oxygen | 15.999 |
| 9 | F | Fluorine | 18.998 |
| 10 | Ne | Neon | 20.18 |
| 11 | Na | Sodium | 22.99 |
| 12 | Mg | Magnesium | 24.305 |
| 13 | Al | Aluminium | 26.982 |
| 14 | Si | Silicon | 28.085 |
| 15 | P | Phosphorus | 30.974 |
| 16 | S | Sulfur | 32.06 |
| 17 | Cl | Chlorine | 35.45 |
| 18 | Ar | Argon | 39.948 |
| 19 | K | Potassium | 39.098 |
| 20 | Ca | Calcium | 40.078 |
| 21 | Sc | Scandium | 44.956 |
| 22 | Ti | Titanium | 47.867 |
| 23 | V | Vanadium | 50.942 |
| 24 | Cr | Chromium | 51.996 |
| 25 | Mn | Manganese | 54.938 |
| 26 | Fe | Iron | 55.845 |
| 27 | Co | Cobalt | 58.933 |
| 28 | Ni | Nickel | 58.693 |
| 29 | Cu | Copper | 63.546 |
| 30 | Zn | Zinc | 65.38 |
| 31 | Ga | Gallium | 69.723 |
| 32 | Ge | Germanium | 72.63 |
| 33 | As | Arsenic | 74.922 |
| 34 | Se | Selenium | 78.971 |
| 35 | Br | Bromine | 79.904 |
| 36 | Kr | Krypton | 83.798 |
| 37 | Rb | Rubidium | 85.468 |
| 38 | Sr | Strontium | 87.62 |
| 39 | Y | Yttrium | 88.906 |
| 40 | Zr | Zirconium | 91.224 |
| 41 | Nb | Niobium | 92.906 |
| 42 | Mo | Molybdenum | 95.95 |
| 43 | Tc | Technetium | 98 |
| 44 | Ru | Ruthenium | 101.07 |
| 45 | Rh | Rhodium | 102.906 |
| 46 | Pd | Palladium | 106.42 |
| 47 | Ag | Silver | 107.868 |
| 48 | Cd | Cadmium | 112.414 |
| 49 | In | Indium | 114.818 |
| 50 | Sn | Tin | 118.71 |
| 51 | Sb | Antimony | 121.76 |
| 52 | Te | Tellurium | 127.6 |
| 53 | I | Iodine | 126.904 |
| 54 | Xe | Xenon | 131.293 |
| 55 | Cs | Caesium | 132.905 |
| 56 | Ba | Barium | 137.327 |
| 57 | La | Lanthanum | 138.905 |
| 58 | Ce | Cerium | 140.116 |
| 59 | Pr | Praseodymium | 140.908 |
| 60 | Nd | Neodymium | 144.242 |
| 61 | Pm | Promethium | 145 |
| 62 | Sm | Samarium | 150.36 |
| 63 | Eu | Europium | 151.964 |
| 64 | Gd | Gadolinium | 157.25 |
| 65 | Tb | Terbium | 158.925 |
| 66 | Dy | Dysprosium | 162.5 |
| 67 | Ho | Holmium | 164.93 |
| 68 | Er | Erbium | 167.259 |
| 69 | Tm | Thulium | 168.934 |
| 70 | Yb | Ytterbium | 173.045 |
| 71 | Lu | Lutetium | 174.967 |
| 72 | Hf | Hafnium | 178.49 |
| 73 | Ta | Tantalum | 180.948 |
| 74 | W | Tungsten | 183.84 |
| 75 | Re | Rhenium | 186.207 |
| 76 | Os | Osmium | 190.23 |
| 77 | Ir | Iridium | 192.217 |
| 78 | Pt | Platinum | 195.084 |
| 79 | Au | Gold | 196.967 |
| 80 | Hg | Mercury | 200.592 |
| 81 | Tl | Thallium | 204.38 |
| 82 | Pb | Lead | 207.2 |
| 83 | Bi | Bismuth | 208.98 |
| 84 | Po | Polonium | 209 |
| 85 | At | Astatine | 210 |
| 86 | Rn | Radon | 222 |
| 87 | Fr | Francium | 223 |
| 88 | Ra | Radium | 226 |
| 89 | Ac | Actinium | 227 |
| 90 | Th | Thorium | 232.038 |
| 91 | Pa | Protactinium | 231.036 |
| 92 | U | Uranium | 238.029 |
| 93 | Np | Neptunium | 237 |
| 94 | Pu | Plutonium | 244 |
| 95 | Am | Americium | 243 |
| 96 | Cm | Curium | 247 |
| 97 | Bk | Berkelium | 247 |
| 98 | Cf | Californium | 251 |
| 99 | Es | Einsteinium | 252 |
| 100 | Fm | Fermium | 257 |
| 101 | Md | Mendelevium | 258 |
| 102 | No | Nobelium | 259 |
| 103 | Lr | Lawrencium | 266 |
| 104 | Rf | Rutherfordium | 267 |
| 105 | Db | Dubnium | 268 |
| 106 | Sg | Seaborgium | 269 |
| 107 | Bh | Bohrium | 270 |
| 108 | Hs | Hassium | 277 |
| 109 | Mt | Meitnerium | 278 |
| 110 | Ds | Darmstadtium | 281 |
| 111 | Rg | Roentgenium | 282 |
| 112 | Cn | Copernicium | 285 |
| 113 | Nh | Nihonium | 286 |
| 114 | Fl | Flerovium | 289 |
| 115 | Mc | Moscovium | 290 |
| 116 | Lv | Livermorium | 293 |
| 117 | Ts | Tennessine | 294 |
| 118 | Og | Oganesson | 294 |
Lists of Elements in Periodic Table
Atomic Number Is The Number Of What
You can also list the elements in various ordered properties with printable tables below.
Lists of Elements by Group Number in Periodic Table
Atomic Number Is The Number Of Protons In An Atom
» Group 1» Group 2» Group 3» Group 4» Group 5» Group 6» Group 7» Group 8» Group 9» Group 10» Group 11» Group 12» Group 13» Group 14» Group 15» Group 16» Group 17» Group 18